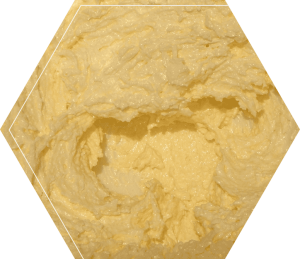
Phenomenon/Problem:
Vigorously shaking heavy whipping cream in a solid container produces butter and buttermilk.
Stimuli: How will students experience and/or observe the phenomenon/problem?
Students vigorously shake heavy whipping cream in mason jars until butter is formed. Or students watch a video of someone relatable doing the same.
Essential Questions:
Why does shaking the cream take it from a homogenous liquid to a liquid and a solid?
Related Phenomena/Problems:
Samples of heavy whipping cream, half n’ half, whole milk, 2% and non-fat milk are compared and contrasted.
Liquid dairy products are centrifuged and compared.
A heterogenous mixture including some form of oil and water are physically mixed together using different strategies.
The solid substance that formed in the mason jar can be touched, smeared, tasted, and evaluated for predicted content.
Heavy whipping cream is tested for the presence of lipids, carbohydrates, and proteins.
Students engineer, prototype, and test a device to homogenize a heterogenous mixture.
Students experiment with substances they predict will serve well as emulsifiers/surfactants for oil/water style mixtures.
Considerations for Instructional Design:
The ability for this phenomenon to be experienced and or replicated by students is unique and contributes to student equity.
Focusing on sense-making will be more important than the associated vocabulary.
Explanation:
Students experience vigorously shaking a mason jar of heavy whipping cream and butter forming.
Depending on the setup of the phenomenon, students may or may not know that the semisolid substance that has settled out of the liquid is butter. In general, the heavy whipping cream was a homogenous mixture of carbohydrates, lipids, protein, some minerals, and water. We don’t typically think of lipids or oils as mixing evenly in water; however, in this scenario, the lipids are surrounded by stabilizing proteins. The stabilizing proteins prevent the lipids from sticking to each other. In other words, the proteins act as an emulsifier.
The kinetic force created by vigorously and continuously shaking the jar causes molecules to collide. This force is enough to disrupt the stabilizing proteins surrounding the lipids. Without the stabilizing proteins, the lipids begin to stick together. Eventually, most of the lipids coalesce into a semisolid glob of butter. (MS-PS1-4) The remaining liquid is known as buttermilk.
Two types of mixtures
Mixtures are combinations of two or more distinct chemical substances. Based on the distribution of their particles, mixtures can be classified as either homogeneous or heterogeneous.
If the particles are evenly mixed, then the substance is considered a homogenous mixture. For example, saltwater is a homogeneous mixture because the sodium and chloride ions are evenly mixed at the molecular level. Such a mixture, called a solution, cannot be separated by filtration, centrifugation, or settlement.
On the other hand, dirt mixed with water is a heterogeneous mixture because its particles are not evenly dispersed. This mixture can also be removed by filtering the water. (MS-PS1-2)
Liquid dairy products are homogenized, but they don’t start that way.
Milk begins as a heterogeneous colloid composed of various molecules dissolved or suspended in water. A colloid is a mixture wherein one substance (in this case, lipids), normally insoluble in another, is suspended as microscopic, dispersed globules throughout the solution (Njoo et al., 2019). (MS-PS1-1) (MS-PS1-4)
Today, customers purchasing dairy prefer a product that is evenly mixed. Raw milk, or milk that has not been homogenized separates relatively quickly, with the cream rising to the top. To remedy this, machines are used to physically homogenize it. The machines typically use a pump and pistons that force the milk back and forth through tiny openings. (Wilbey, 2016) This action physically separates the globules into fine-sized droplets. (MS-PS3-1) Protein molecules in the milk, especially casein, surround the tiny droplets and stabilize the lipids/keep them from sticking together. This reduces the surface tension interfering with the attractive forces of the lipids. Before milk itself is homogenized, the cream layer is separated from the raw milk. The milk is then homogenized, and the cream can be homogenized using the same process.
Making butter from cream requires force/energy:
Mentioned above was the kinetic energy created by vigorously shaking the container with the heavy whipping cream. Shaking causes more collisions of molecules with each other and the edges of the container being used. The proteins surrounding and stabilizing the lipids in the cream (allowing for a homogenized mixture) become separated from the lipid molecules. Without this stabilization, the lipid molecules begin to stick together and separate out.
In addition to the physical forces, there are two other forces at play. First, when the lipid molecules are no longer stabilized, they are repelled by water, which is a polar molecule with a slight charge on one side. This charge is created by the hydrogen protons being angled, meaning the side of the molecule that is a further distance from the oxygen molecule, has a slight negative charge while the opposite side has a slight positive charge. (MS-PS2-3) (HS-PS1-3) Lipids are a nonpolar molecule and are repelled by polar molecules. Similarly, there are some weak forces between the lipid molecules that can cause them to be attracted to each other.
(Lower, Claire. 2022)
The third force plays a very tiny role but is nonetheless present. This force is intermolecular, or between the lipid molecules themselves. Intermolecular forces are technically known as Van de Waal forces, of which there are three types. In the case of lipids, they are London Dispersion forces, which occur in non-polar molecules and are created by the constant motion of electrons. At any given point, it’s possible, even for a nonpolar molecule to have a very slight charge (have a dipole) and therefore have weak attraction between molecules. London dispersion forces vary and increase with the amount of surface area and the size of the molecules. Molecules with more surface area occupy more space, increasing the chances of electrons interacting. Larger molecules have more electrons, which creates increased opportunities for dipoles to be created. (Anderson, Paul. 2014) (UK Essays. 2019) (MS-PS1-1) (MS-PS2-3) (HS-PS-1) (HS-PS1-3) As a side note, London Dispersion forces prevent butter from melting in the fridge. (MS-PS1-4)
Student and teacher questions about this phenomenon/problem that could be instructionally productive:
What is milk made of?
Is the cream part of the milk to begin with?
Does the milk’s richness depend on the type of cow? Like it’s age, etc.
What’s the difference between skim milk and 2%?
Why does it separate?
What is the glob of stuff?
Is the glob of stuff just fat?
Was there a chemical change?
Explaining the phenomenon/problem or related phenomena could lead students toward developing the following DCIs:
PS1.A: Structure and Properties of Matter
Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms. (MS-PS1-1)
Each atom has a charged substructure consisting of a nucleus, which is made of protons and neutrons, surrounded by electrons. (HS-PS1-1) Building toward looking at the chemical structures of the organic proteins and how the arrangement of the atoms influences polarity.
Each pure substance has characteristic physical and chemical properties (for any bulk quantity under given conditions) that can be used to identify it. (MS-PS1-2), (MS-PS1-3) Building towards as students identify the butter and its properties. Additionally, in looking at the chemical structures of lipids and proteins.
Gases and liquids are made of molecules or inert atoms that are moving about relative to each other. (MS-PS1-4) Building toward the polar and nonpolar repulsion between lipids and water based on polarity, the emulsifying properties presented by protein molecules, and the intermolecular London dispersion forces between molecules of butter.
In a liquid, the molecules are constantly in constant with others; in a gas, they are widely spaced except when they happen to collide. In a solid, atoms are closely spaced and may vibrate in position but do not change relative to locations (MS-PS1-4) Build toward the understanding of London dispersion forces.
The changes of state that occur with variations in temperature or pressure can be described and predicted using these models of matter. (MS-PS1-4) Building toward in some capacity when understanding the physical processes of homogenization. Additionally, temperate can be varied, and the rate or lack of state changes can vary.
High School: The structure and interactions of matter at the bulk scale are determined by electrical forces within and between atoms. (HS-PS1-3) (secondary to HS-PS2-6) Building toward the effects of polarized molecules and London dispersion forces.
PS2.B: Types of Interactions
Electric and magnetic (electromagnetic) forces can be attractive or repulsive, and their sizes depend on the magnitudes of the charges, currents, or magnetic strengths involved and on the distances between interaction objects. (MS-PS2-3) Building toward understanding the requirements for lipids to emulsify into a homogenous mixture, the forces between water and lipids, and the intermolecular London dispersion forces.
Attraction and repulsion between electric charges at the atomic scale explain the structure, properties, and transformations of matter, as well as the contact forces between material objects. (secondary to HS-PS-1) (secondary to HS-PS1-3) Building toward understanding the roles polar and nonpolar molecules play in this phenomenon as well as the London dispersion forces.
PS3.A: Energy
Motion energy is properly called kinetic energy; it is proportional to the mass of the moving object and grows with the square of its speed. (MS-PS3.1) Built toward shaking the jar of heavy whipping cream vigorously and the resulting formation of butter, a semi-solid after imposing molecular collisions at high speeds.
Notes about relevance and authenticity (funds of knowledge, interests, identity) Why might students be engaged?
Students likely have experience with butter as a food product.
If butter can be created in class, every student gets to experience the phenomenon first-hand. Additionally, they can touch, taste, and test the actual products.
This is a unique context for exploring the science of mixtures.
Seeing the timelapse of non-homogenized/pasteurized milk will be new and unique to most students.
Resources/References
Economic Research Service, Rhodes, M. T., Kuchler, F., McClelland, K., & Hamrick, K. S., Consumer Food Safety Practices: Raw Milk Consumption and Food Thermometer Use (n.d.).
Fellows, P.J.. Food Processing Technology: Principle and Practice. Fourth Edition. 2017.
Helmenstine, Anne. Surfactant Definition and Examples. November 21, 2023. Available: https://sciencenotes.org/surfactant-definition-and-examples/.
Is homogenized milk good for you? (2014, March 23). Dairy Moos. https://www.dairymoos.com/is-homogenized-milk-good-for-you/
Lower, Claire. Cream Science: On Whipping, Butter, and Beyond. October 18, 2022. Serious Eats. Accessed May 17, 2023. Available: https://www.seriouseats.com/the-science-of-whipped-cream-butter-creme-fraiche.
National Dairy Council. (2014, December 2). Homogenization 101: What does it mean and what is homogenized milk? Undeniably Dairy.
Njoo, D., Lakshmidevi, P., Chen, A., & Chen, Al. (2019, July). Using centrifugation of milk to teach composition and physical and chemical properties. Dairy Discoveries. https://drive.google.com/file/d/1X-iXCnbY2XBJBcze7NLN92h_i_dWScz0/view
Oner Ceylan, Tulay Ozcan. Effect of the cream cooling temperature and acidification method on the crystallization and textural properties of butter, LWT, Volume 132, 2020,109806. ISSN 0023-6438. https://doi.org/10.1016/j.lwt.2020.109806.